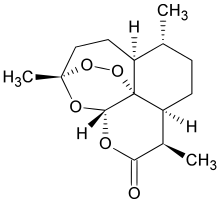
ARTEMISININ
.......................
http://www.google.com/patents/WO2012154906A1?cl=en
To a solution of compound 10 (7 g, 15.4 mmol) and ammonium molybdate (1 .51 g, 7.7 mmol) in t-BuOH (60 mL) was added 50% H202 (10 mL, 150 mmol). The solution was stirred with additional H2O2 (5 ml_, 75 mmol) added every 12 hours. At 72 hours, the mixture was diluted with water (100 mL), extracted with CH2CI2 (3x100 mL), dried over MgS04, filtered and concentrated. The yellow crude mixture was dissolved in DCM (50 mL) and treated with p-toluensulfonic acid (pTSA) (285 mg, 1 .5 mmol). The resulting solution was stirred for 72 hours before being concentrated and filtered through a plug of silica (hexanes/diethyl ether 10:1 eluent). The resulting yellow oil could be purified by flash chromatography (ethyl acetate in hexanes 0% to 20%), or recrystallized from heptane to obtain 1 .26 g artemisinin (1 ) (29% yield).
[0058] NOTE: Amberlite IR120 hydrogen form and other homogenous proton sources could also be used in place of p-toluenesulfonic acid.
[0059] IR (film) v/cm"1 2956 (m), 2933 (m), 2884 (m), 2861 (m), 1739 (s), 1201 (m), 1 1 14 (s), 1033 (m), 1028 (m), 995 (s), 883 (m). [a]D 20 = +64.0 (c 1 .20, CHCI3) (nat.
[G]D 20 = +66.6 (c 0.90, CHCI3)). 1H NMR (400 MHz, CDCI3) δ 5.84 (s, 1 H), 3.38 (dq, J = 7.4, 5.5 Hz, 1 H), 2.41 (ddd, J = 14.4, 12.9, 3.9 Hz, 1 H), 2.06-1 .92 (m, 2H), 1 .90- 1 .82 (m, 1 H), 1 .79-1 .70 (m, 2H), 1 .52-1 .31 (m, 3H), 1 .42 (s, 3H), 1 .18 (d, J = 7.4 Hz, 3H), 1 .10-1 .00 (m, 2H), 0.98 (d, J = 5.9 Hz, 3H). 13C NMR (100 MHz, CDCI3) δ
172.7, 106.0, 94.3, 80.1 , 50.7, 45.6, 38.2, 36.5, 34.2, 33.5, 25.8, 25.5, 24.0, 20.5, 13.2. HRMS calculated for Ci5H2205Na [M+ Na] 305.1365, found 305.1356.
.....................
HSQC
COSY
HMBC
////
No comments:
Post a Comment