Scheme
1. Diene-Transmissive Diels–Alder Cycloaddition Sequences of [3]- and
[4]Dendralene with the Prototypical Olefinic Dienophile
The
first synthesis of all five possible monomethylated [4]dendralenes has
been achieved via two distinct synthetic strategies. The Diels–Alder
chemistry of these new dendralenes (as multidienes) with an electron
poor dienophile, N-methylmaleimide (NMM), has been studied. Thus,
simply upon mixing the dendralene and an excess of dienophile at
ambient temperature in a common solvent, sequences of cycloadditions
result in the rapid generation of complex multicyclic products. Distinct
product distributions are obtained with differently substituted
dendralenes, demonstrating that dendralene substitution influences the
pathway followed, when a matrix of mechanistic possibilities exists.
Dendralene site selectivities are traced to electronic, steric and
conformational effects, thereby allowing predictive tools for
applications of substituted dendralenes in future synthetic endeavors.
Scheme 2. Diene-Transmissive Diels–Alder Cycloaddition Sequences of [4]Dendralene (1) with the Dienophile N-Methylmaleimide (NMM)
Scheme 3. Syntheses of the Five Mono-Methyl-Substituted-[4]Dendralenes
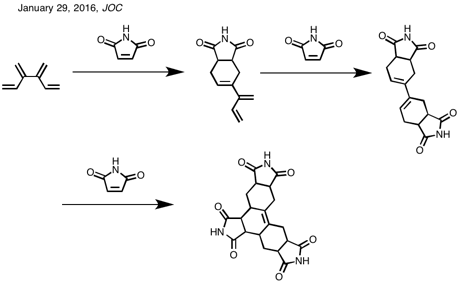
FROM https://naturalproductman.wordpress.com/2016/01/29/11137/
3 Diels-Alder reactions in 1 go

FROM https://naturalproductman.wordpress.com/2016/01/29/11137/
Tris-adduct 36
An analytic sample of 36 was obtained by recrystallization from EtOAc/hexane to give colorless needles, mp 255–257 °C; Rf 0.20 (EtOAc, 100%);
1H NMR (300 MHz, CDCl3) δ 3.22 (dd, J = 8.6, 5.9 Hz, 1H), 3.19–3.07 (m, 3H), 3.04–2.91 (m, 5H), 2.90 (s, 6H), 2.86 (s, 3H), 2.65 (ddd, J = 14.1, 13.4, 5.4 Hz, 1H), 2.35 (ddd, J = 14.3, 5.0, 2.5 Hz, 1H), 2.16–2.05 (m, 2H), 2.03–1.91 (m, 1H), 1.85–1.74 (m, 1H), 1.54 (d, J = 6.8 Hz, 3H) ppm;
13C NMR (75 MHz, CDCl3)
δ 179.7 (C), 178.5 (C), 178.4 (C), 178.3 (C), 177.0 (C), 176.6 (C),
130.8 (C), 130.8 (C), 44.4 (CH), 43.4 (CH), 40.8 (CH), 40.6 (CH), 40.3
(CH), 39.2 (CH), 38.8 (CH), 33.7 (CH), 29.0 (CH), 25.0 (CH3), 24.9 (CH3), 24.8 (CH3), 24.7 (CH2), 24.4 (CH2), 23.1 (CH2), 16.5 (CH3) ppm;
IR (KBr disc) νmax = 2961, 2948, 2842, 1770, 1695, 1435, 1383, 1286 cm–1;
LRMS (70 eV, EI) m/z (%) 453 ([M]+•, 100%), 438 (7), 342 (33), 256 (14), 112 (39);
HRMS calc for C24H27N3O6 [M]+• 453.1900, found 453.1905.
Synthesis and Diels–Alder Reactivity of Substituted [4]Dendralenes
† Research
School of Chemistry, The Australian National
University, Canberra, ACT 2601, Australia
‡ School
of Chemistry, The University of New South
Wales, Sydney, NSW 2052, Australia
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.5b02583
Publication Date (Web): January 12, 2016
Copyright © 2016 American Chemical Society
*E-mail: michael.sherburn@anu.edu.au.
ACS Editors' Choice - This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes.













No comments:
Post a Comment