
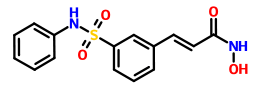
Belinostat (PXD101)
FAST TRACK FDA , ORPHAN STATUS
PXD101;PX105684;PXD
UNII:F4H96P17NZ
N-Hydroxy-3-(3-phen
N-HYDROXY-3-[3-[(PH
NSC726630
(E)-N-hydroxy-3-[3-
414864-00-9 [RN]
866323-14-0 [RN]
Beleodaq®
CLIP
SYNTHESIS AND SPECTRAL DATAJournal of Medicinal Chemistry, 2011 , vol. 54, 13 pg. 4694 - 4720
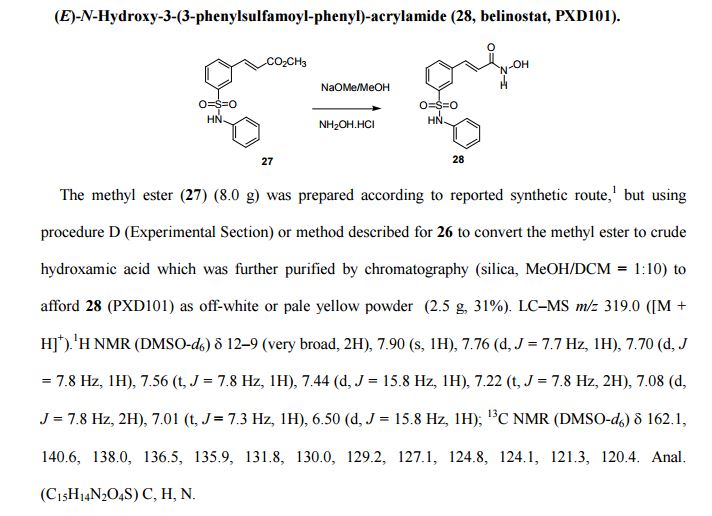
(E)-N-Hydroxy-3-(3-phenylsulfamoyl-phenyl)-acrylamide (28, belinostat, PXD101).
http://pubs.acs.org/doi/full/10.1021/jm2003552
http://pubs.acs.org/doi/suppl/10.1021/jm2003552/suppl_file/jm2003552_si_001.pdf
The methyl ester (27) (8.0 g) was prepared according to reported synthetic route,
(Watkins, C. J.; Romero-Martin, M.-R.; Moore, K. G.; Ritchie, J.; Finn, P. W.; Kalvinsh, I.;
Loza, E.; Dikvoska, K.; Gailite, V.; Vorona, M.; Piskunova, I.; Starchenkov, I.; Harris, C. J.;
Duffy, J. E. S. Carbamic acid compounds comprising a sulfonamide linkage as HDAC
inhibitors. PCT Int. Appl. WO200230879A2, April 18, 2002.)
but using procedure D (Experimental Section) or method described for 26 to convert the methyl ester to crude
hydroxamic acid which was further purified by chromatography (silica, MeOH/DCM = 1:10) to
afford 28 (PXD101) as off-white or pale yellow powder (2.5 g, 31%).
LC–MS m/z 319.0 ([M +H]+).
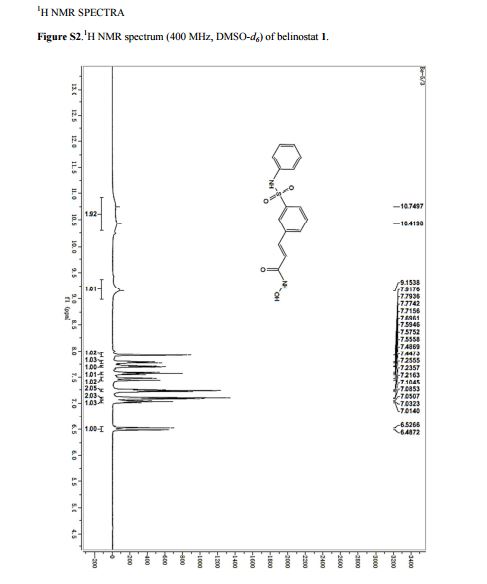
1H NMR (DMSO-d6) 12–9 (very broad, 2H), 7.90 (s, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.70 (d, J
= 7.8 Hz, 1H), 7.56 (t, J = 7.8 Hz, 1H), 7.44 (d, J = 15.8 Hz, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.08 (d,J = 7.8 Hz, 2H), 7.01 (t, J = 7.3 Hz, 1H), 6.50 (d, J = 15.8 Hz, 1H);
13C NMR (DMSO-d6) 162.1, 140.6, 138.0, 136.5, 135.9, 131.8, 130.0, 129.2, 127.1, 124.8, 124.1, 121.3, 120.4.
Anal.
(C15H14N2O4S) C, H, N
Identifications:
1H NMR (Estimated) for Belinostat

Experimental: 1H NMR (300 MHz, DMSO-d6): δ 6.52 (d, J=15.9 Hz, 1H), 6.81–7.12 (m, 6H), 7.33 (d, J=15.9 Hz, 1H), 7.47–7.67 (m, 3 H), 7.87 (s, 1H), 9.00–11.20 (br, 3H).
SEE COMPILATION ON SIMILAR COMPOUNDS AT ..............http://drugsynthesisint.blogspot.in/p/nostat-series.html
| 1H NMR (Estimated) for Belinostat |

Experimental: 1H NMR (300 MHz, DMSO-d6): δ 6.52 (d, J=15.9 Hz, 1H), 6.81–7.12 (m, 6H), 7.33 (d, J=15.9 Hz, 1H), 7.47–7.67 (m, 3 H), 7.87 (s, 1H), 9.00–11.20 (br, 3H).
SEE COMPILATION ON SIMILAR COMPOUNDS AT ..............http://drugsynthesisint.blogspot.in/p/nostat-series.html
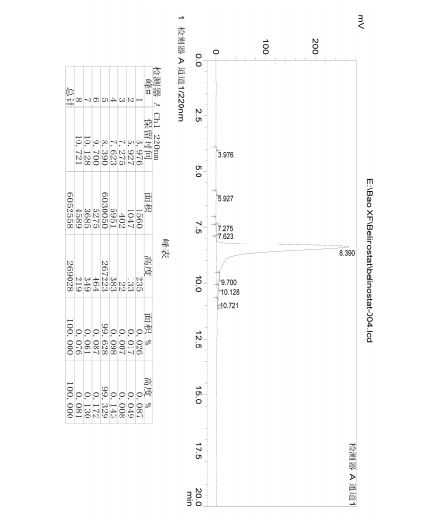
HPLC
NMR PREDICT
1H NMR PREDICT
13C PREDICT
////////////Belinostat, PXD101, novel HDAC inhibitor, Beleodaq, Folotyn, Spectrum Pharmaceuticals, Inc., Henderson, Nevada, Istodax, Celgene Corporation, Summit, New Jersey, CuraGen Pharma, FDA 2014
O=S(=O)(Nc1ccccc1)c2cc(\C=C\C(=O)NO)ccc2
SEE COMPILATION ON SIMILAR COMPOUNDS AT ..............http://drugsynthesisint.blogspot.in/p/nostat-series.html

NMR PREDICT
1H NMR PREDICT
13C PREDICT
////////////Belinostat, PXD101, novel HDAC inhibitor, Beleodaq, Folotyn, Spectrum Pharmaceuticals, Inc., Henderson, Nevada, Istodax, Celgene Corporation, Summit, New Jersey, CuraGen Pharma, FDA 2014
O=S(=O)(Nc1ccccc1)c2cc(\C=C\C(=O)NO)ccc2
SEE COMPILATION ON SIMILAR COMPOUNDS AT ..............http://drugsynthesisint.blogspot.in/p/nostat-series.html








No comments:
Post a Comment