endo-(phenylsulfonyl)bicyclo[2.2.1]hept-5-ene-2-carbonitrile :
Freshly distilled cyclopentadiene (2.369 mL, 29.02 mmol) was added to a cooled (ice bath, 0 °C) solution of compound 122a (1.21 g, 5.8 mmol) in anhydrous CH2Cl2 (20 mL). The reaction mixture was stirred for 3 h at 0 °C, then the volatiles were evaporated and the oily residue was purified by flash column chromatography (SiO2, petrolether/EtOAc 3:2) to afford exo 123b isomer (eluted first, 223 mg, 18 %, white solid) and endo 123a isomer (eluted second, 1.21 g, 81%, white solid).
Analytical data for end isomer: 1H NMR (500 MHz, CDCl3): δ = 7.89-7.87 (m, 2H), 7.71-7.68 (m, 1H), 7.62-6.59 (m, 1H), 6.33 (ddd, J = 22.2, 5.6, 3.0 Hz, 2H), 3.81 (dd, J = 5.3, 3.2 Hz, 1H), 3.40 (m, 1H), 3.34 (m, 1H), 2.70 (dd, J = 22.2, 5.3, 2.0 Hz, 1H), 1.73 (ddd, J = 9.6, 3.7, 1.8 Hz, 1 H), 1.66 (dm, J = 9.6 Hz, 1 H) ppm.
13C NMR (126 MHz, CDCl3): δ = 139.58, 135.63, 134.62, 134.57, 129.99, 128.17, 120.20, 70.00, 49.37, 48.89, 45.67, 32.18. ppm.
Spectral data were identical with those reported in the literature.
Bradley, P. J., Grayson, D. H. J. Chem. Soc. Perkin. Trans. 1. 2002, 1794.
///////////////////
O=S(=O)(c1ccccc1)C3C(C#N)C2C=CC3C2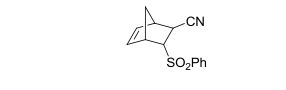
No comments:
Post a Comment