Tofogliflozin
- Molecular FormulaC22H26O6
- Average mass386.438 Da
NMR

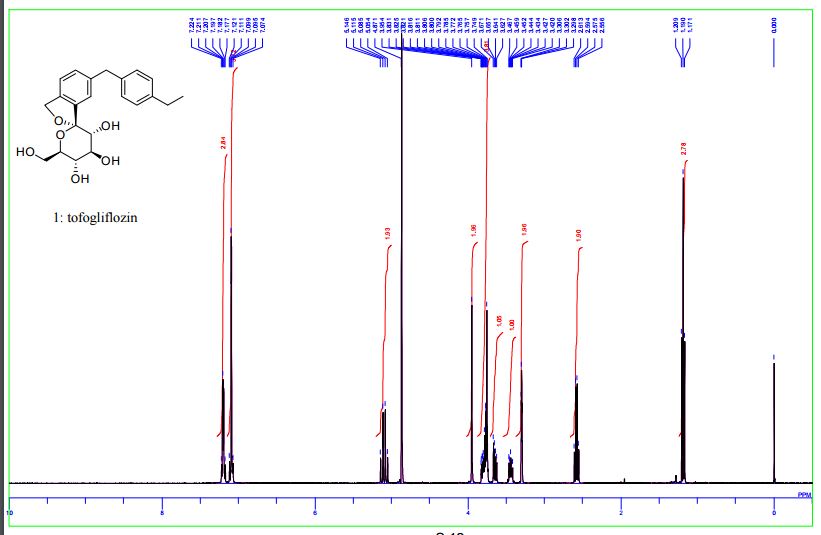
1 H-NMR (CD 3 OD) δ: 1.19 (3H, t, J = 7.5Hz), 2.59 (2H, q, J = 7.5Hz) ,3.42-3 .46 (1H , m), 3.65 (1H, dd, J = 5.5,12.0 Hz) ,3.74-3 .82 (4H, m), 3.96 (2H, s), 5.07 (1H , d, J = 12.8Hz), 5.13 (1H, d, J = 12.8Hz) ,7.08-7 .12 (4H, m) ,7.18-7 .23 (3H, m) .
MS (ESI +): 387 [M +1] +.
MS (ESI +): 387 [M +1] +.
Second set
J. Med. Chem., 2012, 55 (17), pp 7828–7840
DOI: 10.1021/jm300884k
1H NMR (400 MHz, CD3OD) δ: 1.20 (3H, t, J = 7.6 Hz), 2.58 (2H, q, J = 7.6 Hz), 3.42–3.47 (1H, m), 3.63–3.67 (1H, m), 3.75–3.88 (4H, m), 3.95 (2H, s), 5.06 (1H, d, J = 12.3 Hz), 5.12 (1H, d, J = 12.5 Hz), 7.07–7.14 (4H, m), 7.17–7.23 (3H, m).
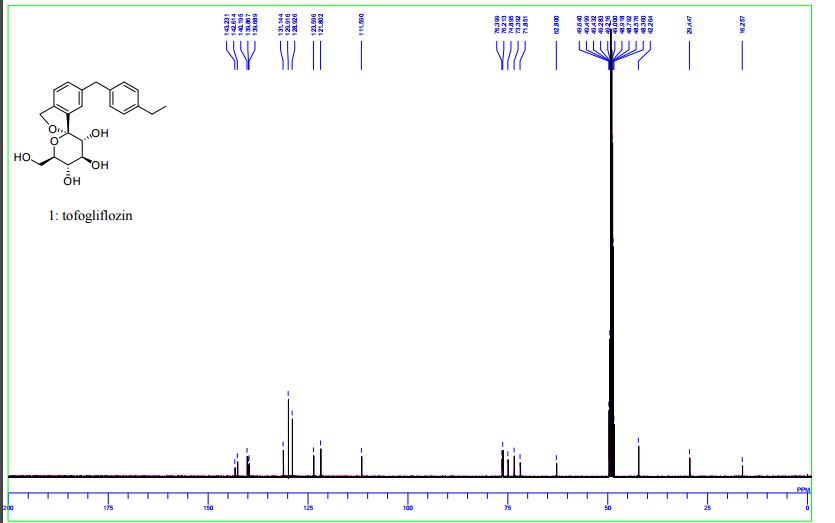
13C NMR (100 MHz, CD3OD) δ: 16.3, 29.4, 42.3, 62.8, 71.9, 73.4, 74.9, 76.2, 76.4, 111.6, 121.8, 123.6, 128.9, 129.9, 131.1, 139.7, 139.9, 140.2, 142.6, 143.2.
MS (ESI): 387 [M + H]+. HRMS (ESI), m/z calcd for C22H27O6 [M + H]+ 387.1802, found 387.1801.
AND
(1S,3′R,4′S,5′S,6′R)-6-[(4-Ethylphenyl)methyl]-6′-(hydroxymethyl)-3′,4′,5′,6′-tetrahydro-3H-spiro[2-benzofuran-1,2′- pyran]-3′,4′,5′-triol (1, tofogliflozin).
To a solution of 17b (89.9 g, 145 mmol) in DME (653 mL) and MeOH (73.0 mL), 2 N NaOH aq. solution (726 mL, 1.45 mol) was added dropwise for 1 h at waterbath temperature. After stirring at rt for 1 h, 2 N H2SO4 aq. solution (436 mL) was added slowly to the mixture. Water (700 mL) was added to the mixture, and the resultant mixture was extracted with AcOEt (500 mL × 2). The resultant organic layer was washed with brine (1.00 L) and then dried over anhydrous Na2SO4 (250 g). The mixture was concentrated in vacuo to obtain 1 (57.3 g, quant) as a colorless amorphous solid;
[α]D 26 +24.2° (c 1.02, MeOH);
1 H NMR (400 MHz, CD3OD) δ: 1.19 (3H, t, J = 7.6 Hz), 2.58 (2H, q, J = 7.6 Hz), 3.42−3.47 (1H, m), 3.63−3.67 (1H, m), 3.75−3.88 (4H, m), 3.95 (2H, s), 5.06 (1H, d, J = 12.5 Hz), 5.12 (1H, d, J = 12.5 Hz), 7.07−7.14 (4H, m), 7.17−7.23 (3H, m);
13C NMR (100 MHz, CD3OD) δ: 16.3, 29.4, 42.3, 62.8, 71.9, 73.4, 74.9, 76.2, 76.4, 111.6, 121.8, 123.6, 128.9, 129.9, 131.1, 139.7, 139.9, 140.2, 142.6, 143.2;
MS (ESI) m/z: 387 [M + H]+ ; HRMS (ESI) calcd for C22H27O6 [M + H]+ 387.1802, found 387.1801
DOI: 10.1021/acs.joc.5b02734 J. Org. Chem. 2016, 81, 2148−2153
Ohtake, Y.; Sato, T.; Kobayashi, T.; Nishimoto, M.; Taka, N.; Takano, K.; Yamamoto, K.; Ohmori, M.; Yamaguchi, M.; Takami, K.; Yeu, S.-H.; Ahn, K.-H.; Matsuoka, H.; Morikawa, K.; Suzuki, M.; Hagita, H.; Ozawa, K.; Yamaguchi, K.; Kato, M.; Ikeda, S. J. Med. Chem. 2012, 55, 7828−7840
Paper
A Scalable Synthesis of Tofogliflozin Hydrate
Pharmaceutical Research Center, Disha Pharmaceutical Group Co., Ltd., Weihai 264205, China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00175, http://pubs.acs.org/doi/abs/10.1021/acs.oprd.6b00175
*E-mail: xiudongyang-disha@hotmail.com.
A newly process for the synthesis of tofogliflozin hydrate, a sodium-glucose cotransporter type 2 (SGLT2) inhibitor, was described. Three improvements were achieved, including the development of a regioselective Friedel–Crafts reaction, a high-yield reduction, and a mild metal–halogen exchange. These improvements ultimately resulted in the isolation of tofogliflozin hydrate as a white solid in >99% purity (HPLC area) and 23% overall yield after 12 steps without column chromatography.
Tofogliflozin hydrate white solid with 99.56% purity by HPLC. Water content: 4.47%.
Mp: 71−80 oC. [α]20 D = +23.9 (c = 1.0, CH3OH).
1H NMR (400 MHz, CD3OD) δ 7.23-7.18 (m, 3H), 7.12-7.08(m, 4H), 5.13 (d, J = 12.4 Hz, 1H), 5.07 (d, J = 12.4 Hz, 1H), 3.96 (s, 2H), 3.83-3.73 (m, 4H), 3.65 (dd, J = 11.9, 5.5 Hz, 1H), 3.41-3.47 (m, 1H), 2.59 (q, J = 7.6 Hz, 2H), 1.19 (t, J = 7.6 Hz, 3H).
13C NMR (100 MHz, CD3OD) δ 143.2, 142.6, 140.2, 139.9, 139.7, 131.2, 129.9, 128.9, 123.6, 121.8, 111.6, 76.4, 76.2, 74.9, 73.4, 71.9, 62.8, 42.3, 29.5, 16.3.
HRMS (ESI) m/z: [M+H]+ Calcd for C22H27O6 387.1802; Found 387.1805.
IR (KBr, cm-1) ν: 3362, 2962, 2927, 1637, 1513, 1429, 1095, 1034, 808, 770. Spectroscopic data were identical with those reported.1b, 2
1. (a) Suzuki, M.; Honda, K.; Fukazawa, M.; Ozawa, K.; Hagita, H.; Kawai, T.; Takeda, M.; Yata, T.; Kawai, M.; Fukuzawa, T.; Kobayashi, T.; Sato, T.; Kawabe, Y.; Ikeda, S. J. Pharmacol. Exp. Ther. 2012, 341, 692.
(b) Ohtake, Y.; Sato, T.; Kobayashi, T.; Nishimoto, M.; Taka, N.; Takano, K.; Yamamoto, K.; Ohmori, M.; Yamaguchi, M.; Takami, K.; Yeu, S.-H.; Ahn. K.-H.; Matsuoka, H.; Morikawa, K.; Suzuki, M.; Hagita, H.; Ozawa, K.; Yamaguchi, K.; Kato, M.; Ikeda, S. J. Med. Chem. 2012, 55, 7828.
(c) Ikeda, S.; Takano, Y.; Cynshi, O.; Tanaka, R.; Christ, A. D.; Boerlin, V.; Beyer, U.; Beck, A.; Ciorciaro, C.; Meyer, M.; Kadowaki, T. Diabetes, Obesity and Metabolism 2015, 17, 984.
2. (a) Murakata, M.; Ikeda, T.; Kimura, N.; Kawase, A.; Nagase, M.; Yamamoto, K.; Takata, N.; Yoshizaki, S.; Takano, K. Crystal of spiroketal derivative, and process for production thereof. European Appl. EP 2308886 A1, April 13, 2011.
(b) Ohtake, Y.; Emura, T.; Nishimoto, M.; Takano, K.; Yamamoto, K.; Tsuchiya, S.; Yeu, S.; Kito, Y.; Kimura, N.; Takeda, S.; Tsukazaki, M.; Murakata, M.; Sato, T. J. Org. Chem. 2016, 81, 2148.
SYNTHESIS...........http://www.allfordrugs.com/2013/12/18/tofogliflozin/
////////////////////////
CLIP
There are two scalable synthetic routes reported to prepare tofogliflozin.2 An efficient production synthesis of tofogliflozin hydrate from alcohol 2 was first described by Murakata et al. (Scheme 1, route 1).2a In 2016, Ohtake et al. reported an improved synthetic route, which achieved in just 7 linear steps (Scheme 1, route 2).2b They selected the optimal protecting groups for the purpose of chemoselective activation and crystalline purification, and obtained the pure tofogliflozin in a good overall yield. However, these methods suffer from several drawbacks. Firstly, some reagents, such as BH3 (Scheme 1, route 2) and 2-Methoxyproene (3, Scheme 1), are toxic or highly volatile. Meanwhile, the use of Palladium reagents may lead to an excess of residual heavy metal in the final product. Secondly, manufacturing costs in these methods are high due to the application of expensive raw materials and reagents. Last but not least, the key tactical stages that involve Br/Li exchange of aryl bromide followed by addition to gluconolactone 5 need the cryogenic conditions (< -60 oC), and this method is not suitable for industrial production. Herein, we report a newly developed synthetic method for tofogliflozin hydrate starting from readily available raw materials and affording good overall yield.
SCHEME 2 FOR
2. (a) Murakata, M.; Ikeda, T.; Kimura, N.; Kawase, A.; Nagase, M.; Yamamoto, K.; Takata, N.; Yoshizaki, S.; Takano, K. Crystal of spiroketal derivative, and process for production thereof. European Appl. EP 2308886 A1, April 13, 2011. (b) Ohtake, Y.; Emura, T.; Nishimoto, M.; Takano, K.; Yamamoto, K.; Tsuchiya, S.; Yeu, S.; Kito, Y.; Kimura, N.; Takeda, S.; Tsukazaki, M.; Murakata, M.; Sato, T. J. Org. Chem. 2016, 81, 2148.





No comments:
Post a Comment