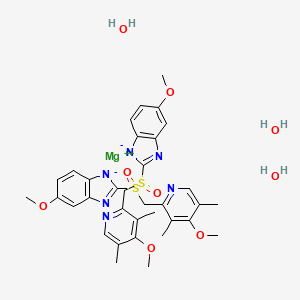
Esomeprazole Magnesium Trihydrate
| Nexium; KS-1054; Esomeprazole magnesium; AC1L2XMA; 217087-09-7; AC-402; | |
| Molecular Formula: | C34H42MgN6O9S2 |
|---|---|
| Molecular Weight: | 767.16708 g/mol |
magnesium;5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]benzimidazol-1-ide;trihydrate
Esomeprazole magnesium dihydrate (CAS 217087-10-0) |
| A proton pump inhibitor | |
| CAS Number: | 217087-10-0 |
| Purity: | ≥98% |
| Molecular Weight: | 749.15 |
| Molecular Formula: | C34H36MgN6O6S2 2H2O |
(−)-enantiomer of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)-methyl]sulfinyl]-1H-benzimidazole, i.e. S-omeprazole.
The compound 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, having the generic name omeprazole, and therapeutically acceptable salts thereof, are described in EP 5129. The specific alkaline salts of omeprazole are disclosed in EP 124 495. Omeprazole is a proton pump inhibitor, i.e. effective in inhibiting gastric acid secretion, and is useful as an antiulcer agent. In a more general sense, omeprazole may be used for prevention and treatment of gastric-acid related diseases in mammals and especially in man.
Omeprazole is a sulfoxide and a chiral compound, wherein the sulfur atom being the stereogenic center. Thus, omeprazole is a racemic mixture of its two single enantiomers, the R and S-enantiomer of omeprazole, herein referred to as R-omeprazole and S-omeprazole. The absolute configurations of the enantiomers of omeprazole have been determined by an X-ray study of an N-alkylated derivative of the (+)-enantiomer in non-salt form. The (+)-enantiomer of the non-salt form and the (−)-enantiomer of the non-salt form were found to have R and S configuration, respectively, and the (+)-enantiomer of the magnesium salt and the (−)-enantiomer of the magnesium salt were also found to have R and S configuration, respectively. The conditions for the optical rotation measurement for each of these enantiomers are described in WO 94/27988.
Esomeprazole sold under the brand name Nexium among others,[1] is a proton pump inhibitor which reduces stomach acid. It is used in the treatment of dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, and Zollinger-Ellison syndrome.
It decreases secretion of acid through inhibition of the H+/K+-ATPase in the parietal cells of the stomach. By inhibiting the functioning of this transporter, the drug prevents formation of stomach acid.
Esomeprazole is the (S)-(−)-enantiomer of omeprazole. Esomeprazole is currently sold over the counter in the US and the UK.
IR in KBr pellets
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent., pics are from free internet space
(1HNMR)
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent., pics are from free internet space
MASS
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent., pics are from free internet space
Factors Influencing the Selectivity in Asymmetric Oxidation of Sulfides Attached to Nitrogen Containing Heterocycles Muthu Seenivasaperumal,a Hans-Jürgen Federsel,b Anne Ertanbc and Kálmán J. Szabóa * a Stockholm University, Arrhenius Laboratory, Department of Organic Chemistry SE-106 91 Stockholm, Sweden; b Global Process R&D and c Early Development, Pharmaceutical and Analytical R&D, AstraZeneca, SE-151 85 Södertälje, Sweden E-mail: kalman@organ.su.se. Fax: +46-8-15 49 08
http://www.rsc.org/suppdata/cc/b7/b700860k/b700860k.pdf
Esomeprazole (5a). This product was prepared according to the above general procedure except that it was isolated as a potassium salt (78% yield and 97% ee) by addition of potassium methoxide to the reaction mixture. The 1 H-NMR data obtained for 5a agrees 3 with the literature values.
8 1 H NMR (DMSO-d6): δ 8.25 (s, 1H), 7.37 (d, 8.6 Hz, 1H), 7.02 (d, 2.6 Hz, 1H), 6.60 (dd, 2.5 Hz, 8.4 Hz, 1H), 4.72 and 4.46 (d (AB system, 12.7 Hz, 2H), 3.75 (s, 3H), 3.70 (s, 3H), 2.21(s, 6H);
13C NMR (DMSO-d6): δ 163.4, 161.8, 153.7, 151.9, 149.1, 147.0, 141.6, 126.5, 124.9, 117.5, 109.0, 99.4, 59.7, 55.2, 48.6, 12.9, 11.3. [α]D 20 = +30.1 (c 1.0, H2O); Lit.8 : [α]D 20 = +30.5 (c 1.0, H2O) for (S), 99.5% ee.
The ee was determined by HPLC (Chiralpak AD-H column, hexane/i-PrOH/AcOH 50:50:0.1, flow rate 0.6 mL/min; tR (minor) = 9.9 min; tR (major) = 11.7 min, λ = 300.3 nm).
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent., pics are from free internet space
(The method used is in accordance with the method described in Example A in WO 96/01623)
Magnesium (0.1 μg, 4.5 mmol) was dissolved and reacted with methanol (50 ml) at 40° C. with a catalytic amount of methylene chloride. The reaction was run under nitrogen and was finished after five hours. At room temperature a mixture of the two enantiomers [90%(−)-isomer and 10%(+)-isomer] of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole (2.84 g, 8.2 mmol) was added to the magnesium methoxide solution. The mixture was stirred for 12 hours whereupon a small amount of water (0.1 ml) was added in order to precipitate inorganic magnesium salts. After 30 minutes stirring, these inorganic salts were filtered off and the solution was concentrated on a rotavapor. The residue was now a concentrated methanolic solution of the enantiomeric mixture (i.e. the title compound contaminated with the (+)-isomer), with an optical purity (enantiomeric excess, e.e.) of 80%. This mixture was diluted with acetone (100 ml) and after stirring at room temperature for 15 minutes, a white precipitate was obtained. Additional stirring for 15 minutes and thereafter filtration afforded 1.3 g (50%) of the title compound as white crystals. Chiral analyses of the crystals and mother liquor were performed by chromatography on an analytical chiral column. The optical purity of the crystals and mother liquor was found to be 98.4 e.e. and 64.4% e.e., respectively. Thus, the optical purity (e.e.) has been enhanced from 80% to 98.4% simply by crystallizing the Mg-salt from a mixture of acetone and methanol. The product was crystalline as shown by powder X-ray diffraction and the magnesium content was 3.44% as shown by atomic absorption spectroscopy. [α]D 20=−131.5° (c=0.5%, methanol).
PATENT

PATENT
| WO1998054171A1 * | May 25, 1998 | Dec 3, 1998 | Astra Aktiebolag | Novel form of s-omeprazole |
| WO2008092939A2 * | Jan 31, 2008 | Aug 7, 2008 | Krka, Tovarna Zdravil, D.D., Novo Mesto | Process for the preparation of optically pure omeprazole via salt formation with a chiral amine or treatment with an entiomer converting enzyme and chromatographic separation |
| WO2009060064A2 * | Nov 7, 2008 | May 14, 2009 | Valpharma S.A. | Pharmaceutical formulations for the oral administration of ppi |
| WO2011144994A1 * | May 20, 2011 | Nov 24, 2011 | Lupin Limited | Pharmaceutical compositions of nsaid and acid inhibitor |
| EP2147918A1 * | Jul 21, 2008 | Jan 27, 2010 | LEK Pharmaceuticals D.D. | Process for the preparation of S-omeprazole magnesium in a stable form |
| US7411070 | Sep 25, 2003 | Aug 12, 2008 | Astrazeneca Ab | Form of S-omeprazole |
| US4255431 | Apr 5, 1979 | Mar 10, 1981 | Aktiebolaget Hassle | Gastric acid secretion inhibiting substituted 2-(2-benzimidazolyl)-pyridines, pharmaceutical preparations containing same, and method for inhibiting gastric acid secretion |
| US4738974 * | Apr 21, 1986 | Apr 19, 1988 | Aktiebolaget Hassle | Base addition salts of omeprazole |
| US4786505 | Apr 20, 1987 | Nov 22, 1988 | Aktiebolaget Hassle | Pharmaceutical preparation for oral use |
| US5530160 | Apr 17, 1995 | Jun 25, 1996 | Rhone-Poulenc Chimie | Process for the preparation of L-aspartic acid from ammonium aspartate |
| US5676884 | Nov 20, 1996 | Oct 14, 1997 | Minnesota Mining And Manufacturing Company | Nonlinear optical materials containing polar disulfone-functionalized molecules |
| US5690960 * | Jul 8, 1994 | Nov 25, 1997 | Astra Aktiebolag | Pharmaceutical formulation of omeprazole |
| US5693818 * | May 27, 1994 | Dec 2, 1997 | Astra Aktiebolag | Process for preparing pure salts of pyridinylmethyl-sulfinyl-1H-benzimidazole |
| US5714504 * | Jan 23, 1995 | Feb 3, 1998 | Astra Aktiebolag | Compositions |
| US5817338 | Jun 7, 1995 | Oct 6, 1998 | Astra Aktiebolag | Multiple unit tableted dosage form of omeprazole |
| US5877192 | Apr 11, 1997 | Mar 2, 1999 | Astra Aktiebolag | Method for the treatment of gastric acid-related diseases and production of medication using (-) enantiomer of omeprazole |
| US5900424 | Jul 8, 1994 | May 4, 1999 | Astra Aktiebolag | Omeprazole magnesium salt form |
| US6369085 | May 5, 1998 | Apr 9, 2002 | Astrazeneca Ab | Form of S-omeprazole |
| US6677455 | Feb 14, 2002 | Jan 13, 2004 | Astrazeneca Ab | Potassium salt of S-omeprazole |
| US6747155 | Apr 1, 2002 | Jun 8, 2004 | Astrazeneca Ab | Process |
| CN1136564A | May 25, 1995 | Nov 27, 1996 | 常州市第四制药厂 | Aomeilazole salt hydrate for gastric acid inhibitor and its preparing method |
| DE4035455A1 | Nov 8, 1990 | May 14, 1992 | Byk Gulden Lomberg Chem Fab | Enantiomerentrennung |
| EP0005129A1 | Apr 3, 1979 | Oct 31, 1979 | Aktiebolaget Hässle | Substituted pyridylsulfinylbenzimidazoles having gastric acid secretion properties, pharmaceutical preparations containing same, and intermediates for their preparation |
| EP0124495A2 | Feb 28, 1984 | Nov 7, 1984 | Aktiebolaget Hässle | Omeprazole salts |
| EP0247983A2 | Apr 16, 1987 | Dec 2, 1987 | Aktiebolaget Hässle | New pharmaceutical preparation for oral use |
| WO1994027988A1 | May 27, 1994 | Dec 8, 1994 | Astra Aktiebolag | Optically pure salts of pyridinylmethyl sulfinyl-ih-benzimidazole compounds |
| WO1995001977A1 | Jul 8, 1994 | Jan 19, 1995 | Astra Aktiebolag | Magnesium omeprazole |
| WO1996001623A1 | Jun 7, 1995 | Jan 25, 1996 | Astra Aktiebolag | Multiple unit tableted dosage form i |
| WO1996002535A1 | Jul 3, 1995 | Feb 1, 1996 | Astra Aktiebolag | Process for synthesis of substituted sulphoxides |
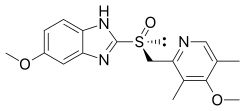 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
(S)-(−)-5-Methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-3H-benzoimidazole
| |
| Clinical data | |
| Pronunciation | /ˌɛsoʊˈmɛprəˌzoʊl, -ˈmiː-, -ˌzɒl/[2] |
| Trade names | Nexium, many others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699054 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50 to 90% |
| Metabolism | Hepatic (CYP2C19, CYP3A4) |
| Biological half-life | 1–1.5 hours |
| Excretion | 80% Renal 20% Faecal |
| Identifiers | |
| CAS Number | 119141-88-7 |
| ATC code | A02BC05 (WHO) |
| PubChem | CID 9579578 |
| DrugBank | DB00736 |
| ChemSpider | 7843323 |
| UNII | N3PA6559FT |
| KEGG | D07917 |
| ChEBI | CHEBI:50275 |
| ChEMBL | CHEMBL1201320 |
| Chemical data | |
| Formula | C17H19N3O3S |
| Molar mass | 345.417 g/mol |
The active ingredient in the proton pump inhibitor NEXIUM® (esomeprazole magnesium) Delayed-Release Capsules for oral administration and NEXIUM (esomeprazole magnesium) For Delayed-Release Oral Suspension is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2pyridinyl)methyl]sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S- and R- isomers. (Initial U.S. approval of esomeprazole magnesium: 2001). Its molecular formula is (C17H18N3O3S)2Mg x 3 H2O with molecular weight of 767.2 as a trihydrate and 713.1 on an anhydrous basis. The structural formula is:
Figure 1
The magnesium salt is a white to slightly colored crystalline powder. It contains 3 moles of water of solvation and is slightly soluble in water. The stability of esomeprazole magnesium is a function of pH; it rapidly degrades in acidic media, but it has acceptable stability under alkaline conditions. At pH 6.8 (buffer), the half-life of the magnesium salt is about 19 hours at 25°C and about 8 hours at 37°C.
NEXIUM is supplied in delayed-release capsules and in packets for a delayed-release oral suspension. Each delayed-release capsule contains 20 mg, or 40 mg of esomeprazole (present as 22.3 mg, or 44.5 mg esomeprazole magnesium trihydrate) in the form of enteric-coated granules with the following inactive ingredients: glyceryl monostearate 40-55, hydroxypropyl cellulose, hypromellose, magnesium stearate, methacrylic acid copolymer type C, polysorbate 80, sugar spheres, talc, and triethyl citrate. The capsule shells have the following inactive ingredients: gelatin, FD&C Blue #1, FD&C Red #40, D&C Red #28, titanium dioxide, shellac, ethyl alcohol, isopropyl alcohol, n-butyl alcohol, propylene glycol, sodium hydroxide, polyvinyl pyrrolidone, and D&C Yellow #10.
Each packet of NEXIUM For Delayed-Release Oral Suspension contains 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of esomeprazole, in the form of the sameenteric-coated granules used in NEXIUM Delayed-Release Capsules, and also inactive granules. The inactive granules are composed of the following ingredients: dextrose, xanthan gum, crospovidone, citric acid, iron oxide, and hydroxypropyl cellulose. The esomeprazole granules and inactive granules are constituted with water to form a suspension and are given by oral,nasogastric, or gastric administration.
////////////CC1=CN=C(C(=C1OC)C)CS(=O)C2=NC3=C([N-]2)C=CC(=C3)OC.CC1=CN=C(C(=C1OC)C)CS(=O)C2=NC3=C([N-]2)C=CC(=C3)OC.O.O.O.[Mg+2]
SLOVENIA
Ljubljana

.jpg)


////////////









No comments:
Post a Comment